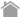Fucci
(Fluorescent Ubiquitination-based Cell Cycle Indicator)
- Real-time visualization of cell-cycle progression
- Spatio-temporal imaging of cell cycle dynamics
- Fluorescent proteins, Green mAG1and Orange mKO2 are used.
- Fucci-G1 Orange labels G1 phase nuclei in orange. Fucci-S/G2/M Green labels S/G2/M phases nuclei in green.
 Fluorescent ubiquitination-based cell cycle indicator (Fucci) is a sophisticated technology which can easily determine G1 and/or S/G2/M phases of the cell cycle. The technology analyzes living cells in a spatio-temporal manner using a dual color scheme of orange and green.
Fluorescent ubiquitination-based cell cycle indicator (Fucci) is a sophisticated technology which can easily determine G1 and/or S/G2/M phases of the cell cycle. The technology analyzes living cells in a spatio-temporal manner using a dual color scheme of orange and green.
Fucci was successfully established by intelligently utilizing ubiquitin-proteasome protein degradation system (awarded the honor of "Top Innovations of 2008”).
 |
Schematic representation of Fucci cell-cycle labeling Fucci-G1 Orange labels G1 phase nuclei in orange. Fucci-S/G2/M Green labels S/G2/M phases nuclei in green. |
Time-lapse imaging of Fucci-G1 Orange & Fucci-S/G2/M Green stable transformants LCV100 microscope (Olympus). Acquisition every 13.5 min. |
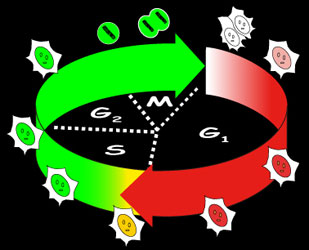
Schematic representation of Fucci constructs
 Fucci is a set of fluorescent probes: Fucci-G1 Orange and Fucci-S/G2/M Green.
Fucci is a set of fluorescent probes: Fucci-G1 Orange and Fucci-S/G2/M Green.
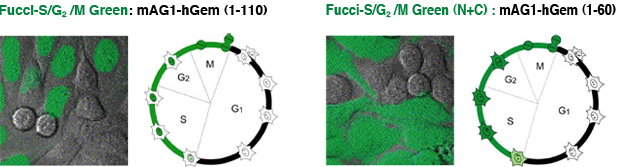
Fucci-G1 Orange is a fusion protein of a fragment of human Cdt1 (amino acids 30-120) with the orange fluorescent mKO2 (monomeric Kusabira-Orange2) that indicates the G1 phase. Fucci-S/G2/M Green is a fusion protein of a fragment of human Geminin (amino acids 1-110) with the green fluorescent protein mAG1 (monomeric Azami-Green1) that visualizes S, G2 and M phases. Fucci-S/G2/M Green (N+C) encodes a fusion protein of a part of human Geminin (1-60) with mAG1.
Terms
Cdt1 :Cdc10 dependent transcript 1 is a conserved replication factor required for licensing the chromosome for a single course of DNA synthesis. Abundantly expressed throughout the cell cycle, Cdt1 is ubiquitinated by the ubiqutin ligase complex SCF skp2 during S and G2 phases and degraded by proteasome.
Geminin: Geminin inhibits the licensing activity of Cdt1. Geminin interferes with the binding of licensing factors to the replication origin once a chromosome has started to replicate during the S phase. During M and G1 phases, Geminin is ubiquitinated by the E3 ligase complex APCcdh1 and degraded by the proteasome.
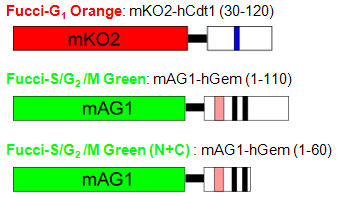
The technology was established by using Amalgaam’s proprietary fluorescent proteins: mAG1 (monomeric Azami-Green1) and mKO2(monomeric Kusabira-Orange2). Both are bright fluorescent proteins and mature rapidly. The graph above shows excitation (dotted line) and emission (solid line) spectra of these proteins.
Provided by Dr. Sakaue-Sawano at RIKEN.
Cell. (2008) 132:487-98.
| Excit./Emiss.Maxima (nm) | Extinction coefficient (M-1cm-1) | Fluorescence quantum yield | pH sensitivity | |
| mAG1 | 492/505 | 55,500 (492 nm) | 0.74 | pKa=5.8 |
| mKO2 | 551/565 | 63,800 (551 nm) | 0.62 | pKa=5.5 |
Cell cycle regulation by E3 ligase
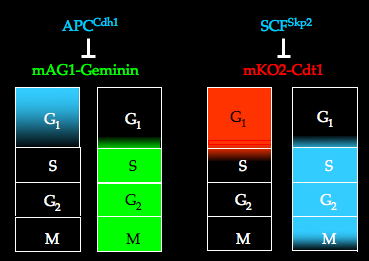 The cell cycle is controlled by ubiqutin-mediated proteolysis. The APCCdh1 (anaphase promoting complex) and SCFSkp2 (SKP-Cullin-F-box) complexes are E3 ligase activities that modify the corresponding target proteins with ubiquitin in a cell cycle-dependent manner. The APCCdh1 complex is active in the late M and G1 phases, while the SCFSkp2 complex is active in the S and G2 phases. The substrates of the APCCdh1 and SCFSkp2 complexes are Geminin and Cdt1 respectively.
The cell cycle is controlled by ubiqutin-mediated proteolysis. The APCCdh1 (anaphase promoting complex) and SCFSkp2 (SKP-Cullin-F-box) complexes are E3 ligase activities that modify the corresponding target proteins with ubiquitin in a cell cycle-dependent manner. The APCCdh1 complex is active in the late M and G1 phases, while the SCFSkp2 complex is active in the S and G2 phases. The substrates of the APCCdh1 and SCFSkp2 complexes are Geminin and Cdt1 respectively.
In the Fucci system, the phases in which Geminin or Cdt1 are not degraded by the proteasome system can be visualized in living cells.
Fluorescent images of Fucci cells
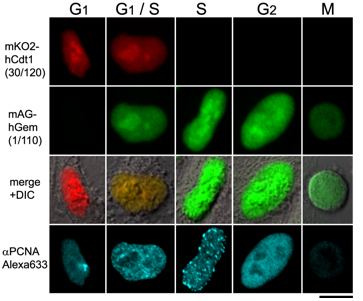 Each cell cycle of the G1, G1/S , S, G2, and M phases can be determined by the combination of Fucci-G1 Orange, Fucci-S/G2/M Green, and an antibody against PCNA.
Each cell cycle of the G1, G1/S , S, G2, and M phases can be determined by the combination of Fucci-G1 Orange, Fucci-S/G2/M Green, and an antibody against PCNA.
G1 phase is indecated by orange. Both orange and green were observed in the G1/S phase. Additional immunostaining color by PCNA was observed at the initiation of the S phase. Cells with pure green fluorescence were either in the S or G2 phase and were distinguished by immunostaining of the S phase. The rest of the cells were classified into the M phase.
These results are consistent with the fact that Cdt1 accumulates in G1, while Geminin accumulates in S/G2/M. PCNA: Proliferation Cell Nuclear Antigen.
Provided by Dr. Sakaue-Sawano at RIKEN.
Cell. (2008) 132:487-98.
Analysis by flow cytometry
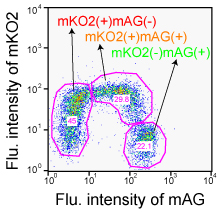 Fucci-expressing HeLa cells were analyzed using BD LSR (Becton Dickinson).
Fucci-expressing HeLa cells were analyzed using BD LSR (Becton Dickinson).
Both mKO2 and mAG were excited by a single 488 nm laser line (Ar). Fluorescence signals were collected at 530 nm (530/28 BP)(FL1) for mAG and at 575 nm (575/26 BP)(FL2) for mKO2. The data were analyzed using FlowJo software (Tree Star).
Provided by Dr. Sakaue-Sawano at RIKEN.
Cell. (2008) 132:487-98.
Cell cycle dependent changes in fluorescence
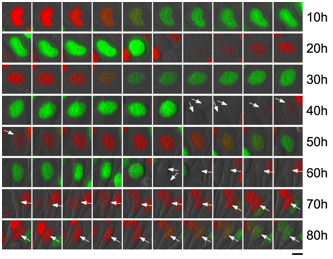
Fucci-expresing HeLa cells: Images acquired every 1 hour.
Arrows indicate cells that were tracked.
Provided by Dr. Sakaue-Sawano at RIKEN.
Cell. (2008) 132:487-98.
1/110 and 1/60 Fucci Green
The difference between these two green probes is the length of the domain. Fucci-S/G2/M Green consists of the amino acids from 1 to 110, while (N+C) is from 1 to 60. (N+C) can visualize not only the cell nucleus but also cytoplasm. We believe that the greatest advantage of the (N+C) probe is the ability to obtain not only the information of the cell cycle but also of the cell morphology.
Provided by Dr. Sakaue-Sawano at RIKEN.
Cell. (2008) 132:487-98.
Chem Biol. (2008)15:1243-8.
We offer antibodies for detection of FUCCI. If you need more information, please click here.
- Anti-monomeric Azami-Green 1 for WB, IP, IC and IH. Code No. PM052M
- Anti-monomeric Kusabira-Orange 2 for WB, IP, IC and IH, Code No. M168-3M
- Anti-monomeric Kusabira-Orange 2 for WB, IP, IC and IH, Code No. PM051M
WB: Western blotting, IP: Immunoprecipitation, IC: Immunocytochemistry, IH: Immunohictochemistry
References
- Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H, Imamura T, Ogawa M, Masai H, Miyawaki A. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 132, 487-498 (2008) PMID: 18267078
- Sakaue-Sawano A, Ohtawa K, Hama H, Kawano M, Ogawa M, Miyawaki A. Tracing the silhouette of individual cells in S/G2/M phases with fluorescence. Chem Biol. 15, 1243-1248 (2008) PMID: 19101468
- Nakayama KI, and Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat. Rev. Cancer 6, 369-381 (2006) PMID: 16633365
- Blow, J. J., and Dutta, A., Preventing re-replication of chromosomal DNA. Nat. Rev. Mol. Cell Biol. 6, 476-486 (2005) PMID: 15928711
- Takeda DY, Parvin JD, Dutta A., Degradation of Cdt1 during S phase is Skp2-independent and is required for efficient progression of mammalian cells through S phase. J. Biol. Chem. 279, 30807-30816 (2004) PMID: 15855168
- Karasawa S, Araki T, Yamamoto-Hino M, Miyawaki A., A green-emitting fluorescent protein from Galaxeidae coral and its monomeric version for use in fluorescent labeling. J. Biol. Chem. 278, 34167-34171 (2003) PMID: 12819206
- Nishitani H, Lygerou Z, Nishimoto T, Nurse P., The Cdt1 protein is required to license DNA for replication in fission yeast. Nature 404, 625-628 (2000) PMID: 10766248
Images and informations provided by
Life Function and Dynamics, ERATO, JST
Laboratory for Cell Function and Dynamics, the Advanced Technology Development Center, the Brain Science Institute, RIKEN
Drs. Sakaue-Sawano Asako, Miyawaki Atsushi
■The images above are taken by using cell lines established in Sakaue-Sawano, A., et al., Cell 132, 487-498 (2008) and not the results by using our products. Please check reference 1. for further information.
■To establish stable cell lines expressing Fucci G1 Orange or Fucci S/G2/M Green, the efficiency of establishment increases by transfecting each genes by expressing vectors which have different drug resistance genes from each other.
Check the PDF for the detailed protocol.
![]() PDF: 96KB
PDF: 96KB
※CoralHue™ fluorescent proteins were co-developed with the Laboratory for Cell Function and Dynamics, the Advanced Technology Development Center, the Brain Science Institute, RIKEN. MBL possesses the license and deals in the products.
Please send us a completed and signed License Acknowledgement.
Please note that the product will be shipped after we receive a
completed and signed License Acknowledgement (PDF file on the right).
Prior to shipping, our staff may contact you to verify the intended use
of the product. Please also note that a separate agreement or a contract
may be required depending on the intended use.
The product you have ordered is sold for research purpose only. It is
not intended for industrial or clinical use, and shall not be used for
any purposes other than research. You are also asked not to hand over or
resell the product to a third party.
If you belong to a profit organization or a business corporation, or
wish to use the product for profit or commercial purposes, please
contact us.

- Fluorescent protein
- Basic fluorescent proteins
- Midoriishi-Cyan [Outline] [Product list]
- Umikinoko-Green [Outline] [Product list]
- Azami-Green [Outline] [Product list]
- Kusabira-Orange [Outline] [Product list]
- dKeima570 [Outline] [Product list]
- Keima-Red [Outline] [Detection of mitophagy with Keima-Red] [Product list]
- Photoconvertible fluorescent proteins
- Dronpa-Green [Outline] [Product list]
- Kaede [Outline] [Product list]
- Kikume Green-Red [Outline] [Product list]
- Organelle targeting vectors [Product list]
- Advanced fluorescent indicators
- Image based Protein-Protein interaction analysis "Fluoppi" [Outline] [Fluoppi Red] [Product list]
- Cell cycle indicator "Fucci" [Outline] [Product list]
- Protein-Protein Interaction Detection System "CoralHue™ Fluo-chase Kit" [Outline] [Product list]
- Anti-CoralHue™ fluorescent protein antibodies
- Anti-CoralHue™ fluorescent protein antibodies [Outline] [Product list]
- Resources
- CoralHue™ vectors full DNA sequence
- Spectrum