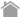- Japan(Japanese / English)
- Global
- MBL TOP
- MBL site search

Fluorescent Staining and Chromogenic Staining
| Fluorescent dyes (small molecular dyes) | FITC (Fluorescein Isothiocyanate), Alexa Fluor® dyes, Cy dyes, etc. |
| Fluorescent proteins | PE (Phycoerythrin), APC (Allophycocyanin), etc. |
■ Chromogenic Staining
| Enzyme labels | Chromogenic substrate |
| Peroxidase (HRP, POD) | DAB, etc. |
| Alkaline phosphatase (ALP, AP) | Fast Red, etc. |
Direct Method, Indirect Method, Amplification Method
■ Direct Method
Using directly labeled fluorescent dyes or enzymes on primary antibodies. Using directly labeled primary antibodies can shorten the experimental time.
■ Indirect MethodUsing secondary antibodies labeled with fluorescent substances or enzymes to detect primary antibodies.
■ Amplification MethodBiotinylated primary antibodies are detected using labeled avidin (biotin-avidin complex). Some manufacturers also offer specialized secondary antibodies for amplification.
| Advantages | Disadvantages | |
| Direct Method |
|
|
| Indirect Method |
|
|
| Amplification Method |
|
|
Types of Tissue Sections
Tissues are fixed with formalin or other fixatives, embedded in paraffin to form tissue blocks, and then cut into sections for staining. These sections have excellent long-term storage stability and is the most commonly used method. However, it takes time for fixation and paraffin embedding, and in the process, the structure of the target protein may change. Therefore, careful consideration of antigen retrieval treatment is crucial during staining.
■ Frozen sectionsFrozen tissues are cut into sections. This method is used for rapid intraoperative pathological diagnosis in clinical settings. Compared to paraffin sections, frozen sections retain the structure and enzymatic activity of target proteins, so no antigen retrieval treatment is required. However, frozen sections are not suitable for long-term storage.
Procedure for FFPE Sections (Indirect Method)
※An example performed at MBL
|
Significance of Each Step
▌ Baking and Deparaffinization
Paraffin is hydrophobic, so if it remains on the tissue, antibodies cannot penetrate the tissue. Therefore, it is necessary to permeate with xylene or ethanol to completely remove the paraffin from the tissue. Since xylene is a hazardous substance, safer alternatives have been developed. Baking treatment helps to melt the paraffin, making it easier to perform the deparaffinization process.
▌ Antigen Retrieval
During formaldehyde fixation and paraffin embedding, antigens in the tissue may become denatured and their epitopes may be hidden. Antigen retrieval exposes the antigens to make them more accessible to antibodies. There are two main methods of antigen retrieval: heat treatment and enzyme treatment. Heat treatment is commonly done using pH 6.0 citrate buffer or pH 9.0 Tris-EDTA buffer. Heat treatment can be done using an autoclave, microwave, pressure cooker, water bath, etc. On the other hand, enzyme treatment involves using proteinase K or pepsin. Care must be taken as tissues tend to lose their structure during enzyme treatment. Retrieval method, pH, temperature, and time should be considered based on the antibody characteristics for optimization.
▌ Inactivation of Endogenous Peroxidase
When performing staining using the enzymatic activity of HRP (POD), it is necessary to remove endogenous peroxidase present in the tissue. This is commonly done by incubating the sections in 3% hydrogen peroxide/PBS for approximately 10 minutes. If endogenous peroxidase inactivation is not adequately performed due to insufficient reaction time or use of inactivated hydrogen peroxide, it might react with peroxidase in the tissue and DAB in the substrate, which lead to non-specific staining.
▌ Counterstaining
Counterstaining is performed to visualize specific structures in the tissue or cells by staining them in contrasting colors. Hematoxylin (blue to purple) or methyl green (green) is commonly used for nuclear staining. After staining with hematoxylin, color development is achieved by using running water or an alkaline aqueous solution. Care should be taken as the color appearance may vary depending on the method and temperature.
▌ Dehydration, Clearing, and Mounting
If using a hydrophobic mounting medium, it is necessary to remove the water contained in the tissue during the previous steps. After dehydration using ethanol, the tissue is permeated with xylene to completely remove the water in the tissue (clearing). This step is not necessary if using a water-soluble mounting medium.
▌ Mounting
The purpose of mounting is to prevent sample drying and fading, and it is necessary for long-term storage. Two types of mounting media are available: water-soluble and non-water-soluble (hydrophobic) media. Xylene-based hydrophobic mounting media are commonly used. To achieve long-term preservation of stained slides, a hydrophobic mounting medium should be selected.
Items needed for experiment (In the case of using FFPE sections)
• Autoclave or microwave (for antigen retrieval)
• Coplin jar
• Humid chamber
• Water-repellent pen (PAP pen)
• FFPE tissue sections
• Xylene (or reagents for deparaffinization)
• Ethanol
• Distilled water
• Antigen retrieval buffer (citric acid buffer, Tris-EDTA buffer, etc.)
• Blocking agent (BSA, serum, etc.)
• PBS
• 0.05-0.2% Tween-20/PBS (PBS-T)
• Primary antibodies
• Enzyme (fluorescent) labeled secondary antibodies
• DAB or other chromogenic substrates
• Mounting medium
Method
※An example performed at MBL
1. Preparation
 Set the incubator to 60°C and place the slides diagonally for paraffin melting. Let them sit for approximately 2 hours.
Set the incubator to 60°C and place the slides diagonally for paraffin melting. Let them sit for approximately 2 hours.
2. Deparaffinization
Permeate with xylene for 5 minutes x 3 times. Then, permeate with anhydrous ethanol for 5 minutes x 1 time, 99.5% ethanol for 5 minutes x 1 time, 90% ethanol for 5 minutes x 1 time, and 70% ethanol for 5 minutes x 1 time. *If paraffin remains after soaking with xylene three times, replace the xylene with new one and soak again.
Permeate with distilled water for 3 minutes x 1 time, and wash with PBS for 2 minutes x 3 times.
*The video has been edited to remove the soaking time.
3. Antigen Retrieval
 Prepare a beaker containing 10 mM citrate buffer (pH 6.0, 0.05% Tween-20) or Tris buffer (pH 9.0, 10 mM Tris-HCl, 0.05% Tween-20, 1 mM EDTA). Autoclave the beaker at 125°C for 5 minutes.
Prepare a beaker containing 10 mM citrate buffer (pH 6.0, 0.05% Tween-20) or Tris buffer (pH 9.0, 10 mM Tris-HCl, 0.05% Tween-20, 1 mM EDTA). Autoclave the beaker at 125°C for 5 minutes.
When the autoclave temperature has dropped to 85°C, remove the beaker and allow it to cool to room temperature.
 Alternatively, perform antigen retrieval using a microwave oven (approximately 10 minutes x 2 times).
Alternatively, perform antigen retrieval using a microwave oven (approximately 10 minutes x 2 times).
Once cool, wash with PBS, add 3% hydrogen peroxide/PBS to a Coplin jar, and incubate for 10 minutes at room temperature.
4. Blocking
 After washing with PBS for 5 minutes x 3 times, use a PAP pen to encircle the tissue section. By using a PAP pen, a hydrophobic barrier can be created around the tissue, which helps save reagents and ensures a uniform reaction.
After washing with PBS for 5 minutes x 3 times, use a PAP pen to encircle the tissue section. By using a PAP pen, a hydrophobic barrier can be created around the tissue, which helps save reagents and ensures a uniform reaction.
※ This step is not mandatory, but it is effective for saving reagents and achieving uniform reaction. This barrier can be removed with xylene.
Add 500 µL blocking buffer (0.5% BSA, 5% goat serum/PBS if the secondary antibody is goat-derived) to the slide, and let it sit for 30 minutes at room temperature.
5. Primary Antibody Reaction
 Remove the blocking buffer and add primary antibody diluted in blocking buffer (e.g., 1 µg/mL) to the slide. Let it sit for 1 hour at room temperature.
Remove the blocking buffer and add primary antibody diluted in blocking buffer (e.g., 1 µg/mL) to the slide. Let it sit for 1 hour at room temperature.
After that, wash with PBS-T (0.05% Tween-20/PBS) for 5 minutes x 3 times, and wash with PBS for 5 minutes x 1 time.

6. Secondary Antibody Reaction and Chromogenic Development
 Add appropriate amount of Histosta™ (Ms + Rb) for Human tissue (Code No. 8460) onto the slide (5-7 drops) and let it sit for 1 hour at room temperature.
Add appropriate amount of Histosta™ (Ms + Rb) for Human tissue (Code No. 8460) onto the slide (5-7 drops) and let it sit for 1 hour at room temperature.
Wash with PBS-T for 5 minutes x 3 times, and wash with PBS for 5 minutes x 1 time.
Add appropriate amount of Histostar™ DAB Substrate Solution (Code No. 8469), and observe the chromogenic development reaction for 3 to a maximum of 10 minutes at room temperature.
*The video has been edited to remove the soaking time.
7. Counterstaining
 Wash with distilled water.
Wash with distilled water.
Add 500 µL Mayer's hematoxylin (or other hematoxylin) to the slide and let it sit for 5 minutes.
Rinse with running water for 20 minutes while taking care to avoid direct water contact with the tissue. Perform color development.
8. Dehydration, Clearing, and Mounting
 Permeate sequentially with 80%, 95%, 99.5% ethanol, and anhydrous ethanol, then permeate with xylene twice.
Permeate sequentially with 80%, 95%, 99.5% ethanol, and anhydrous ethanol, then permeate with xylene twice.
Use a xylene-based hydrophobic mounting medium to mount and dry.