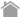- Japan(Japanese / English)
- Global
- MBL TOP
- MBL site search
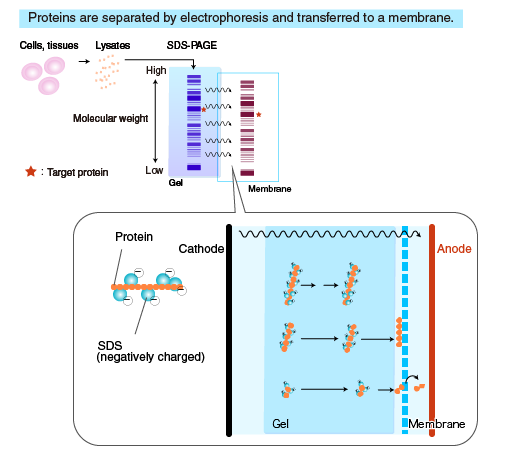
In Western blotting (WB), target proteins are transferred to a hydrophobic membrane after SDS-PAGE and detected using specific antibodies.
The principle
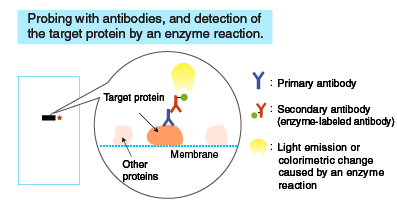
After SDS-PAGE, a membrane is placed on the gel, to which the separated proteins in the gel are electrophoretically transferred. The membrane with transferred proteins is then probed with a primary antibody (an antibody specific for the target protein), washed, and reacted with a secondary antibody labeled with an enzyme, such as horseradish peroxidase (HRP). The bound enzyme activity is used to detect the target protein and visualized by a chemiluminescent or chromogenic method.
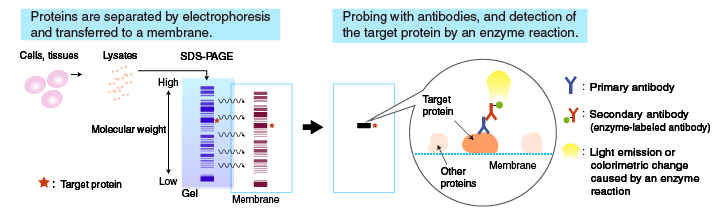
 The flow of the Western blotting procedure is summarized in the diagram on the right. The following sections describe the procedure starting from electrotransfer of proteins to the membrane.
The flow of the Western blotting procedure is summarized in the diagram on the right. The following sections describe the procedure starting from electrotransfer of proteins to the membrane.
Follow the links below for the methods used to generate antibodies, and the principle and method of SDS-PAGE.
>> How to generate antibodies
>> The principle and method of polyacrylamide gel electrophoresis (SDS-PAGE)
Transfer to a membrane
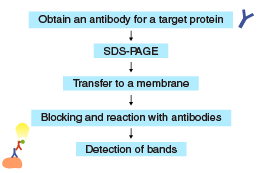
Proteins separated by SDS-PAGE are “transferred” from the polyacrylamide gel to a membrane, using a specialized apparatus (blotting apparatus).
Blotting apparatus
A semi-dry or a tank system can be used for transfer.
| Semi-dry system | Tank system | |
|---|---|---|
| Blotting time | Short | Long |
| Buffer volume | Small | Large |
| Transfer efficiency | Poor for high molecular weight proteins | Good for high molecular weight proteins Evenly transferred |
Membrane
Nitrocellulose and PVDF membranes are commonly used.
Though more expensive, the PVDF membrane is stronger and has a high absorption capacity with proteins, which makes it suitable for CBB staining, detection with certain substrates for alkaline phosphatase, and re-probing.
Nitrocellulose membrane is more fragile but less expensive than PVDF, and does not require pre-wetting with methanol or cause as much non-specific binding.
※Re-probing: Stripping the membrane of antibodies after detection, and probing with another antibody.
Transfer buffer
Different buffers are used for transfer, depending on the molecular weight and the nature of the target proteins. The composition of a commonly used transfer buffer is shown below.
|
25 mM Tris-HCl 192 mM glycine 20% MeOH |

When transferring high molecular weight proteins, reduce the methanol concentration to allow the gel to swell slightly, which facilitates protein transfer.
Blocking and probing with antibodies
Blocking
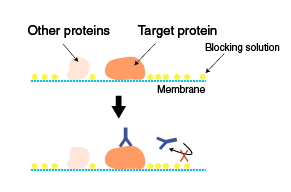 The membrane with transferred proteins is blocked to prevent non-specific binding of antibodies to the membrane.
The membrane with transferred proteins is blocked to prevent non-specific binding of antibodies to the membrane.
※Blocking only prevents non-specific binding, and does not reduce cross-reactivity (specific binding of antibodies to non-target proteins containing the same epitope as found on the target protein).
Various solutions are used for blocking. Commonly used blocking solutions are described below.
1-3% BSA in PBS-T
Bovine serum albumin (BSA) is used as a blocking agent.
1-5% skim milk in PBS-T
Skim milk is more effective in blocking and used frequently. However, it can interfere with specific antigen-antibody reactions.
Skim milk contains the phosphoprotein casein, and therefore is not suitable for the detection of phosphoproteins.
※PBS-T (PBS-Tween 20)
Phosphate-buffered saline (PBS) containing 0.05% Tween 20
Probing with antibodies

Following blocking, the membrane is probed with a primary antibody, and then with a secondary antibody.
The concentration of the primary antibody is critical. If it’s too low, the target protein cannot be detected. If it’s too high, non-specific reactions occur, resulting in detection of non-target proteins.
Determining an optimal concentration of the primary antibody
When using an antibody for the first time, choose a concentration based on the literature or stated on the product datasheet.
If desired results are not obtained, test several different dilutions of the primary antibody, or try a different dilution buffer.
Secondary antibody
Commercially available HRP- or alkaline phosphatase (AP)-labeled secondary antibodies are commonly used.
Check the isotype of the primary antibody and the animal species in which the primary antibody is raised. Care should be taken to avoid using an unsuitable secondary antibody.

[Related topic] HRP-DirecT series -high sensitivity HRP-labeled antibodies-
Detection of bands
Chemiluminescence method
A reaction mixture containing a substrate is added to the membrane. The enzyme attached to the antibody catalyzes a reaction that emits light, which is detected by X-ray film or a cooled CCD camera.
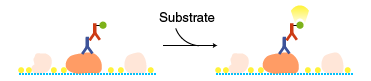
Colorimetric method
A chromogenic substrate is used as a detection reagent. Diaminobenzidine (DAB) or TMB are commonly used as substrates of HRP, and a mixture of BCIP and NBT is used as a substrate of alkaline phosphatase (AP).
Procedure
| Step-by-step procedure | |
|---|---|
| Soak a piece of PVDF membrane slightly larger than the gel in methanol for 1 minute (pre-wetting), and then equilibrate in transfer buffer. Also equilibrate two pieces of filter paper slightly larger than the PVDF membrane in transfer buffer. ※There is no need for pre-wetting a nitrocellulose membrane. Skip using methanol, and equilibrate the membrane in transfer buffer. |
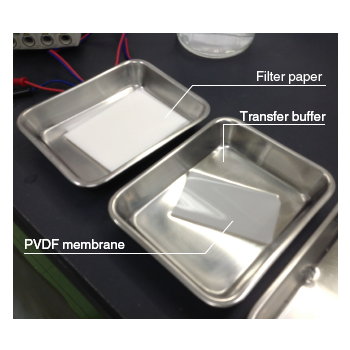 |
| Equilibrate the gel in transfer buffer after electrophoresis. |  |
Place a piece of equilibrated filter paper on the anode plate, and place the membrane, gel, and another piece of filter paper without any air bubbles.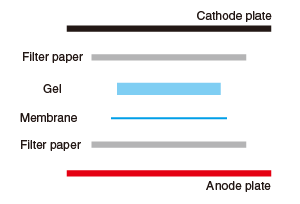 |
 
|
| Connect the plus electrode to the anode plate and connect the negative electrode to the cathode plate. Turn on the power supply and transfer for 1 hour at 50 mA (for one gel)*. *Transfer conditions are different depending on experimental conditions. |
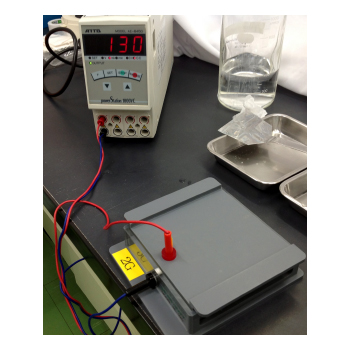 |
| Turn off the power supply, remove the membrane, and temporarily stain to check the efficiency of transfer and to visualize the molecular weight markers to confirm their positions. Ponceau S is commonly used for this purpose because the membrane is easily destained, and it does not interfere with subsequent probing with antibodies. |
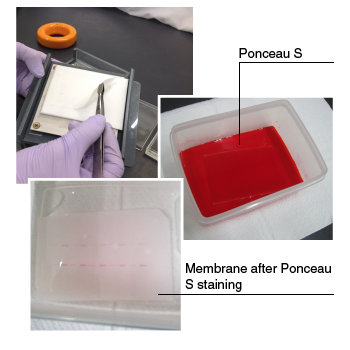 |
![]()
| Step-by-step procedure | |
|---|---|
| Place the membrane in blocking buffer and incubate at room temperature for 1 hour (or at 4°C overnight) with shaking. |
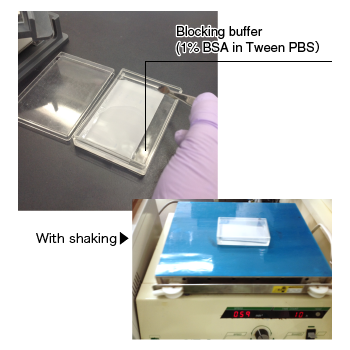 |
| Wash the membrane with washing buffer 3 times (5 minutes each time). | |
| Place the membrane in primary antibody solution diluted in 1% BSA in Tween PBS, pH 7.2, and incubate at room temperature for 1 hour with shaking. |  |
| Wash the membrane with washing buffer 3 times (10 minutes each time). | |
| Place the membrane in secondary antibody diluted in 1% BSA in Tween PBS, pH 7.2, and incubate at room temperature for 1 hour with shaking. |  |
| Wash the membrane with washing buffer 3 times (10 minutes each time). | |
| Chemiluminescence method Prepare the chemiluminescence detection reagent according to the instruction manual. Place the membrane on a piece of plastic wrap, and add the reagent to the membrane. Allow it to stand for 1 minute, and remove the reagent using a pipette. Wrap the membrane in a piece of plastic wrap, remove any air bubbles, and place it under a chemiluminescence detection apparatus (e.g., CCD camera) for viewing. |
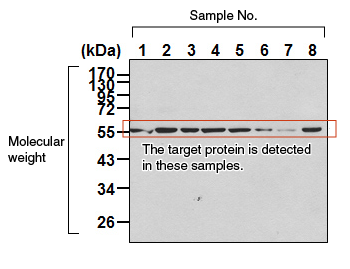 |
| Primary antibodies suitable for Western blotting (WB) (excluding anti-tag antibodies) |
List of monoclonal primary antibodies List of polyclonal primary antibodies |
| Anti-tag antibodies suitable for Western blotting (WB) | List of monoclonal antibodies for tags List of polyclonal antibodies for tags |
| Secondary antibodies suitable for Western blotting (WB) | List of HRP-labeled secondary antibodies |
| Control antibodies | List of antibodies for loading control |
| When blocking | Bovine Serum Albumin Reagent Grade Powder (pH7.0) |
| For shorter processing time! HRP direct-labeled antibodies |
HRP DirecT series These antibodies are high-sensitivity, HRP-labeled antibodies requiring a reaction time as short as 30 minutes. Non-specific signals caused by secondary antibodies are also eliminated. List of HRP direct-labeled anti-tag antibodies List of HRP direct-labeled antibodies (excluding anti-tag antibodies) |