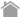- Japan(Japanese / English)
- Global
- MBL TOP
- MBL site search
Human Intestinal Organoid Culture Process
1. Establishment of human intestinal organoids
1-1. Operation
① Lyse human intestinal epithelial cells in a 37°C water bath. |
 |
② Add 10 mL of Basal medium in a 15 mL tube and add ①. |
 |
③ Centrifuge (400 g × 3 min. at room temperature). |
 |
④ Remove the supernatant of the culture medium (after confirming that cells have become pellets). |
|
⑤ Centrifuge at 400 g for 3 minutes at room temperature. |
|
⑥ Remove the remaining medium with a pipette (please be careful that no cells are being removed). |
|
⑦ Add 1 mL of Basal medium to suspend the cells. |
|
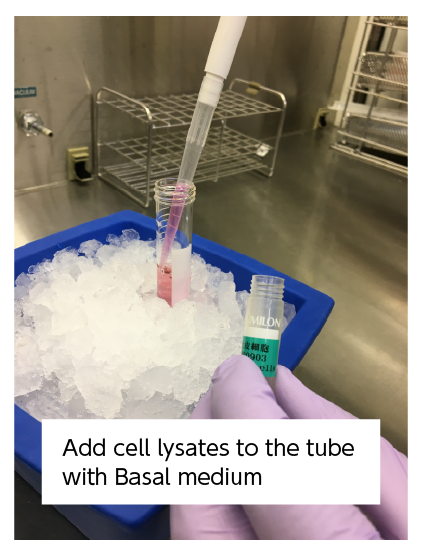
⑧ Collect a portion of the cell suspension, mix it with an equal volume of Trypan Blue Solution to stain the cells, and count the number of viable cells. |
 Images of viable (white) and dead (dark blue) cells |
⑨ To seed 10,000-60,000 cells per well of a 24-well plate, collect necessary cells from ⑦ into a 1.5 mL tube and place the tube on ice. |
⑩ Add Matrigel® to the cell suspension from ⑨ to prepare a cell density of 200,000-1,200,000 cells/mL (10,000-60,000 cells/50 µL). |
|
⑪ Make a dome by seeding 50 µL in each well of 24-well plate. |
⑫ Warm up in a 37°C incubator for 10-15 minutes to gelatinize. |
 |
⑬ Add 500 µL/well of Organoid Growth Medium and incubate in an incubator (add slowly along the wall so that the Matrigel® does not collapses). |
|
⑭ Change medium with Organoid Growth Medium every 2-3 days. |
|
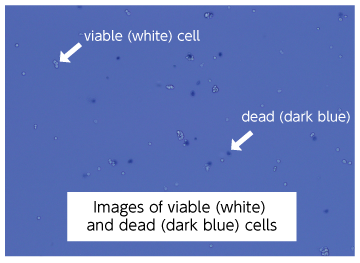
1-2. Example of establishment of intestinal organoids from human intestinal epithelial cells
The following figures are microscopic images (2x objective lens, scale bar = 100 µm) at day 6 and day 10 of culture
(seeding density: 30,000 cells/50 µL)
- ・Overview
- ・Method
1. Establishment of human intestinal organoids
2. Human intestinal organoid passaging(enzymatic dispersion)
3. Frozen stock production of human intestinal organoids
4. Resuscitation of frozen human intestine organoid - ・Related products