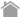- Japan(Japanese / English)
- Global
- MBL TOP
- MBL site search
Quant Tetramer Kit Starter Guide
Products lineup
| Product name | Code No. | ||
|---|---|---|---|
| PE-labeled | APC-labeled | BV421-labeled | |
| QuickSwitch™ Quant HLA-A*01:01 Tetramer Kit | TB-7308-K1 | TB-7308-K2 | TB-7308-K4 |
| QuickSwitch™ Quant HLA-A*02:01 Tetramer Kit | TB-7300-K1 | TB-7300-K2 | ― |
| QuickSwitch™ Quant HLA-A*03:01 Tetramer Kit | TB-7306-K1 | TB-7306-K2 | TB-7306-K4 |
| QuickSwitch™ Quant HLA-A*11:01 Tetramer Kit | TB-7304-K1 | TB-7304-K2 | TB-7304-K4 |
| QuickSwitch™ Quant HLA-A*24:02 Tetramer Kit | TB-7302-K1 | ― | TB-7302-K4 |
| QuickSwitch™ Quant H-2Kb Tetramer Kit | TB-7400-K1 | TB-7400-K2 | TB-7400-K4 |
Kit components
| Component name | Description | Size | Storage | |
|---|---|---|---|---|
| QuickSwitch™ Tetramer | Exiting Peptide loaded MHC tetramer | 500 µL | 2-8°C | |
| Peptide Exchange Factor | The proprietary peptide exchange factor | 13 µL | -20°C | |
| Magnetic Capture Beads | Magnetic beads conjugated with a capture antibody specific for tetramers | 500 µL | 2-8°C | |
| Exiting Peptide Antibody-FITC (25x) | FITC conjugated antibody reacting against Exiting Peptide | 25 µL | 2-8°C | |
| Reference Peptide 1 mM | The peptide for a positive control | 13 µL | -20°C | |
| Assay Buffer (10x) | Washing/Dissolving buffer | 1.7 mL | 2-8°C | |
Materials required but not supplied
| ■ Peptides for new specificity tetramers | ■ Flow cytometer |
| ■ Magnetic tray for microplate | ■ Vortex |
| ■ Microtubes | ■ Round or conical bottom microplates |
| ■ Micropippettes and disposable tips | ■ Aluminum foil |
| ■ Ultra pure water | ■ DMSO |
| ■ Plate shaker (Labline model 4625 or equivalent) | |
| ■ Sonicator (Branson Ultrasonic Cleaner Model #B200 or equivalent) |
WARNINGS AND PRECAUTIONS
- The Reference Peptide and concentrated Assay Buffer (10x) must be brought to room temperature (20-25°C) before use.
- When Assay Buffer (10x) is stored at 2-8°C, some reversible precipitation or turbidity may appear. Incubation at 37°C for a few minutes prior to use is recommended to re-solubilize salts.
- Diluted solutions (antibody and assay buffer) have to be used on the same day as they are prepared. Therefore it is advised to prepare the exact required volumes just before using them.
- QuickSwitch™ Tetramer and Exiting Peptide Antibody are light sensitive and therefore should be protected from light during storage and during all the steps of the assay.
- Care should be taken to avoid splashing and well crosscontaminations.
- All solutions contain sodium azide (≤0.09%) as preservative. If skin or eye contact occurs, wash excessively with water.
- This current protocol uses a magnet to pellet the beads. It is possible to pellet by centrifugation using a plate holder or by suction using filter plates.
Workflow
A Generation of New Specificity Tetramer Using Peptide Exchange
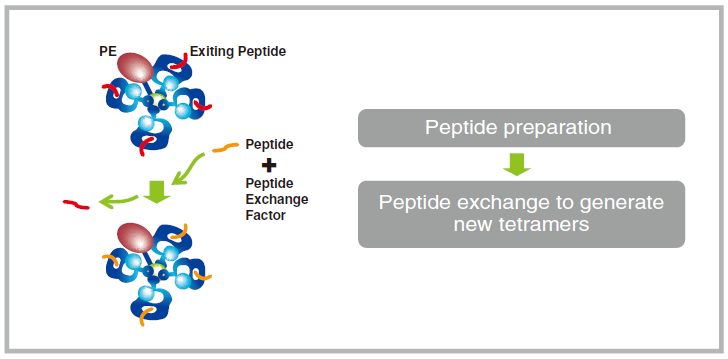
B Quantification of Peptide Exchange Using Flow Cytometric Sandwich Immunoassay
Protocol
A Generation of New Specificity Tetramer Using Peptide Exchange
1 Dissolve each lyophilized peptide to be assayed in DMSO to a 10 mM solution.
(For high affinity peptides, a 1 mM stock solution is a reasonable starting concentration for the assay.)
2 Pipet 50 µL of QuickSwitch™ Tetramer into a microtube.
3 Add 1 µL of peptide and mix gently with pipetting.
4 Add 1 µL of Peptide Exchange Factor and mix gently with pipetting.
5 Repeat steps 1 - 4 for each additional peptide.
6 Prepare Reference Peptide as follows;
(1) Pipet 50 µL of QuickSwitch™ Tetramer into a microtube.
(2) Add 1 µL of Reference Peptide (1 mM) and mix gently with pipetting.
(3) Add 1 µL of Peptide Exchange Factor and mix gently with pipetting.
7 Incubate at least for 4 hours* at room temperature protected from light.
(*Reaction time varies depending on the kit. For details, please refer to the datasheet for each kit.)
8 Refrigerate tetramers at 2-8°C protected from light when not used.
B Quantification of Peptide Exchange Using Flow Cytometric Sandwich Immunoassay
The following assay measures the percentage of original peptide replaced by a competing peptide to help determine whether the resulting tetramer is suitable for antigen-specific CD8+ T cell staining. All control samples are required for the assay. The QuickSwitch™ Calculator on the website can be downloaded for determining percentages of peptide exchange.
WEB
http://www.mblintl.com/quickswitch-peptide-exchange-calculator/
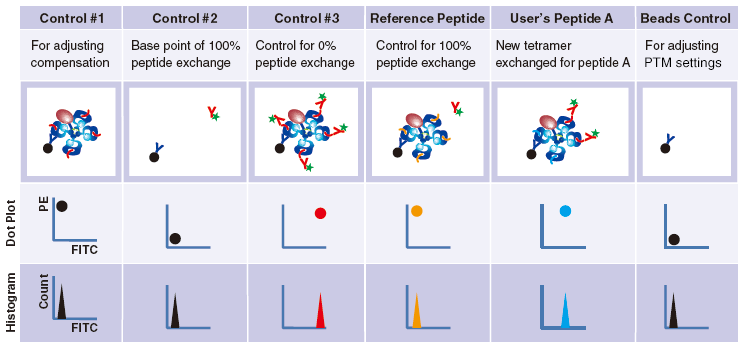
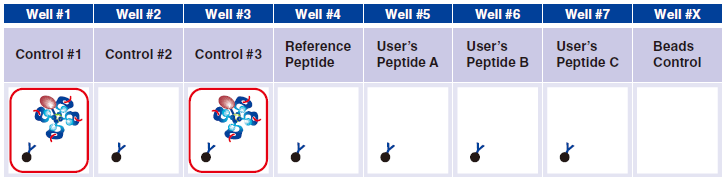
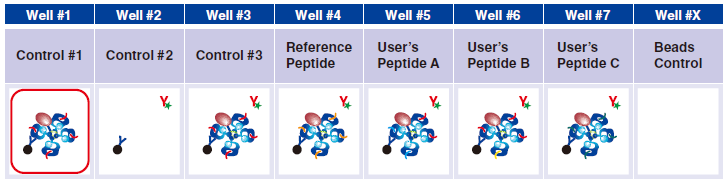
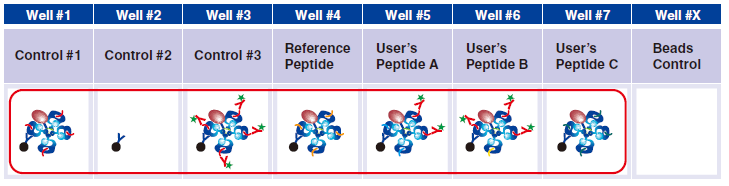
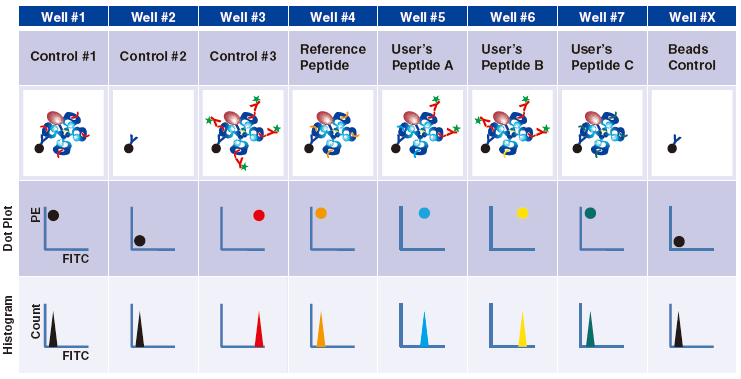
Control #3: Beads that have captured QuickSwitch™ Tetramer. FITC conjugated antibody reacts against Exiting Peptide.
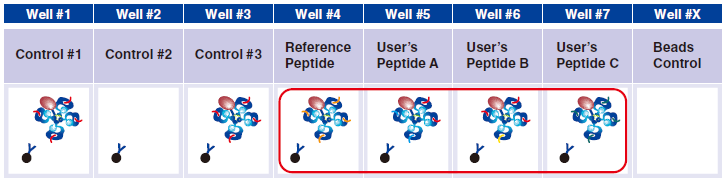
Reference Peptide: This peptide typically undergoes about 100% exchange. FITC conjugated antibody does not react against Reference Peptide.
User’s Peptide: Peptides selected by users. The figure above shows the flow cytometry data for 50% peptide exchange.
Preparation of 1x Assay Buffer
1 Prepare 1x Assay Buffer as follows:
For 1-5 peptide exchanges, prepare 7.5 mL by mixing 750 µL of 10x concentrated Assay Buffer with 6.75 mL of distilled water.
For 6-8 exchanges, double the volumes.
Dispensing Capture Beads
2 Immediately before use, vortex the tetramer capture beads for 30 seconds, followed by a 30-second sonication in a water bath sonicator. If no sonicator is available, vortex an additional 30 seconds.
3 Prepare a round or conical-bottom 96 well microtiter plate.
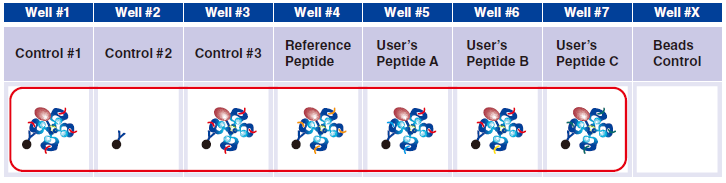
4 Pipet 20 µL Magnetic Capture Beads into each of wells #1-#7. Pipet the beads into wells for three essential
controls, Reference Peptide, and tetramers generated in Step A. The below figure shows the case when three new tetramers are used.
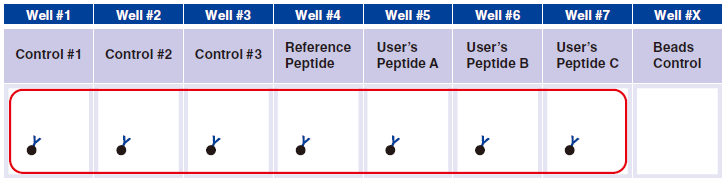
Tetramer Capture
5 Pipet 5 µL 1x Assay Buffer in well #2.
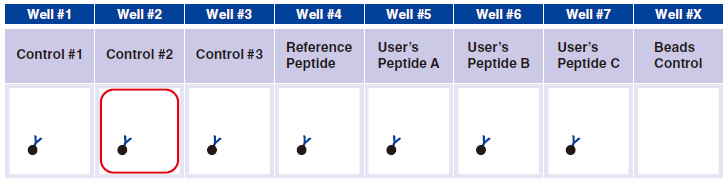
6 Pipet 5 µL QuickSwitch™ Tetramer in wells #1 and #3.
7 In wells #4-#7, pipet 5 µL taken from the difference peptide exchange microtubes as described in Step A.
8 Shake plate for 45 min. at 550 rpm, protected from light with an opaque cover such as a piece of aluminum foil.
Rinse
9 Dispense 150 µL of 1x Assay Buffer in wells #1-#7.
10 Place the plate on a plate magnet for 5 min. to sediment beads.※
11 Flick the plate held tightly to the magnet and blot on a paper towel to minimize cross-contamination of wells.
12 After returning the plate upright, vortex for 2 seconds to disperse the beads.
※It is possible to pellet by centrifugation using a plate holder at 1,000 x g for 5 min.
Preparation of 1x Exiting Peptide Antibody
13 Dilute 25x Exiting Peptide Antibody to 1x as follows: Determine the number (n) of samples to stain with the antibody, including control #2, control #3, Reference Peptides and tetramers generated in Step A. Add one (+1), to account for pipetting errors. In a microtube, pipet (n+1) x 24 µL of Assay Buffer and then add (n+1) x 1 µL of Exiting Peptide Antibody-FITC. Mix by pipetting.
Beads incubation with Exiting Peptide Antibody
14 Pipet 25 μL of 1x Exiting Peptide Antibody-FITC prepared in step 13 in wells #2-#7.
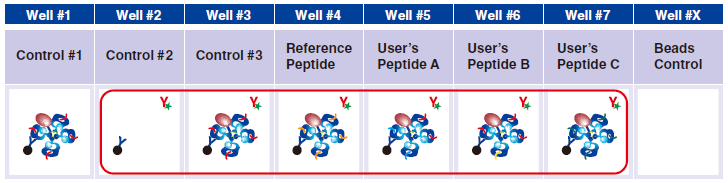
15 Pipet 25 µL of 1x Assay Buffer in well #1.
16 Shake plate for 45 min. at 550 rpm, protected from light.
Rinse
17 Dispense 150 µL of 1x Assay Buffer in wells #1-#7.
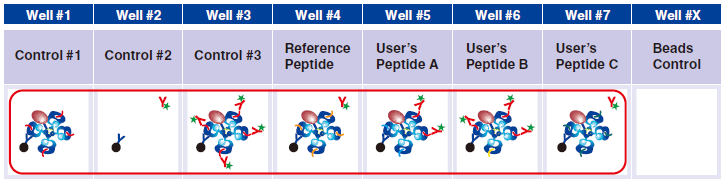
18 Place the plate on a plate magnet for 5 min. to sediment beads.※
19 Flick the plate held tightly to the magnet and blot on a paper towel to minimize cross-contamination of wells.
20 After returning the plate upright, vortex for 2 seconds to disperse the beads. Then remove the plate from the magnet.
21 Resuspend beads in 200 µL 1x Assay Buffer.
※It is possible to pellet by centrifugation using a plate holder at 1,000 x g for 5 min.
Preparing Beads Control for flow cytometry assay
1 Pipet 200 µL 1x Assay Buffer and 5 µL Magnetic Capture Beads into well #X.
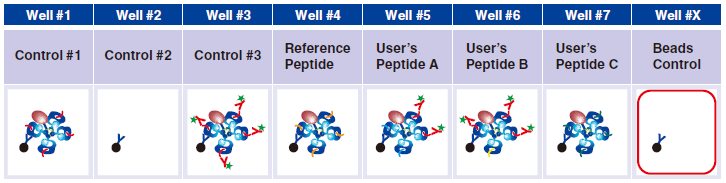
Now they are ready to be analyzed by flow cytometer.
Flow cytometry analysis
For more detailed information, please see the product datasheet.
Determining Percentages of Peptide Exchange
The QuickSwitch™ Calculator can be downloaded for determining percentages of peptide exchange.
Please visit the website at https://www.mblintl.com/quickswitch-peptide-exchange-calculator/.
Tetramers bind to T cell receptors (TCR) via three MHC/peptide monomers. Therefore the minimal recommended peptide exchange percentage should be 75%.