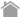- Japan(Japanese / English)
- Global
- MBL TOP
- MBL site search
Four ways to reduce non-specific reactions
・Remove the Fc region of the antibody.
--- Methods to remove the Fc region before labeling (Example 1 for reduction of non-specific reactions)
・Include antibodies against heterophilic antibodies (HAMA Blocker, etc.).
--- Example 2 for reduction of non-specific reactions
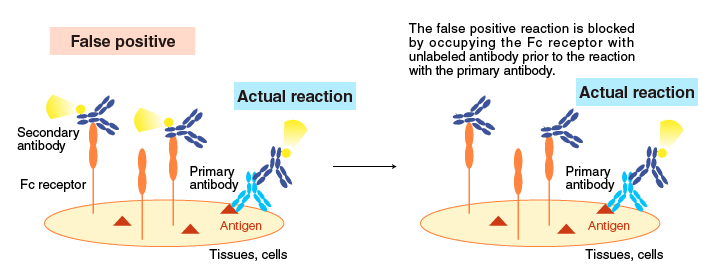
・Pre-mix the sample with IgG from the animal species in which the secondary antibody is produced.
・Use a polyclonal secondary antibody preabsorbed with serum of the same animal.
<Reference> How to label antibodies
Methods to remove the Fc region before labeling
Below are the methods to remove the Fc region of antibodies before labeling. Non-specific reactions caused by Fc receptors are eliminated by using a labeled secondary antibody without the Fc region. One of the following two methods can be used, depending on the molecular weight of the label.
・Labeling SH groups after pepsin digestion
・Labeling NH2 groups after papain digestion
Method 1. Pepsin digestion (for labeling SH groups)
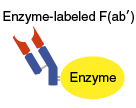 In this method the pFc′ fragment is removed by pepsin digestion, followed by labeling
In this method the pFc′ fragment is removed by pepsin digestion, followed by labeling
the SH groups in the F(ab') fragment. This method is commonly used because the
antibody-binding site will not be blocked by the label.
First, the Fc fragment of IgG is removed by pepsin digestion. The resulting F(ab′)2 fragment is isolated by gel filtration chromatography.
Next, the isolated F(ab′)2 fragment is reduced by 2-mercaptoethylamine (2MEA) treatment to cleave the F(ab′)2 fragment at the hinge region into two F(ab') fragments. An enzyme label is added to the F(ab') fragment (the SH type modification), and gel filtration chromatography is used to separate enzyme-labeled F(ab') from unlabeled F(ab') and the unreacted enzyme.
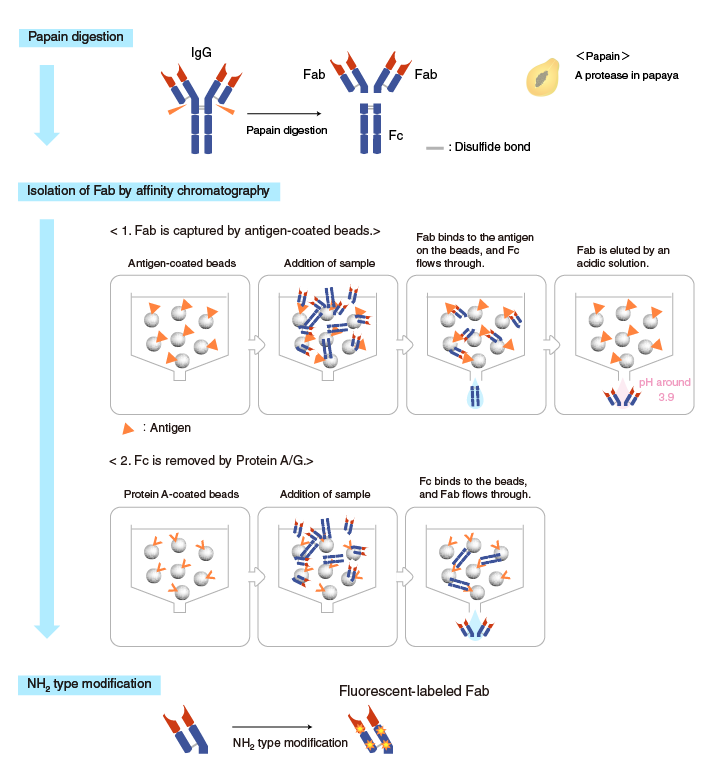
Method 2. Papain digestion (for labeling NH2 groups)
 In this method the Fc region is removed by papain digestion, followed by labeling
In this method the Fc region is removed by papain digestion, followed by labeling
NH2 groups.
First, IgG is digested by papain into Fab and Fc fragments, and then the Fab fragments are isolated by affinity chromatography using one of the following two methods. The first is to use antigen-coated beads to capture the Fab fragments. The second is to use Protein A (or Protein G) to remove the Fc fragment.
Subsequently, a fluorophore is added to the isolated Fab (NH2 type modification), and the reaction results in fluorescent-labeled Fab.
Examples for reduction of non-specific reactions
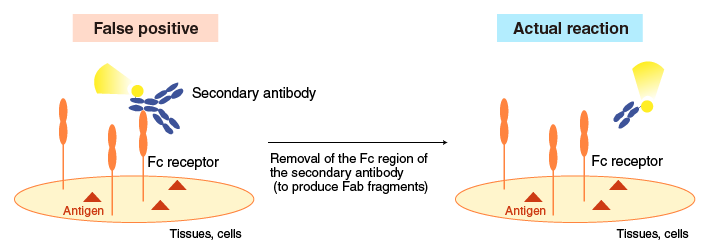
Example 1 for reduction of non-specific reactions
Removal of the Fc region of a secondary antibody (to produce Fab fragments) reduces false positive results caused by Fc receptor binding to the Fc region.
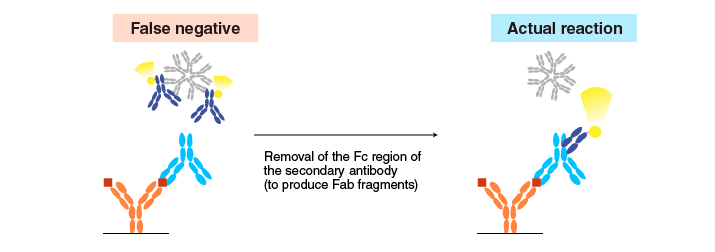
Removal of the Fc region of a secondary antibody (to produce Fab fragments) reduces false negative results caused by Fc-specific antibodies.
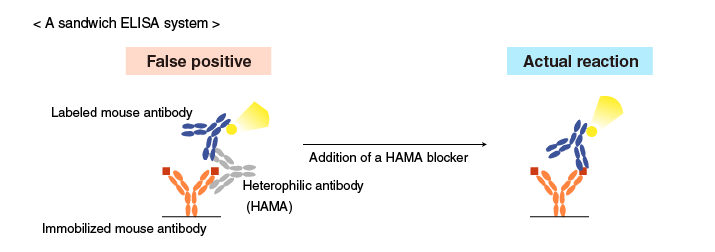
Example 2 for reduction of non-specific reactions
False positive reactions caused by heterophilic antibodies (human anti-mouse antibody, or HAMA) are reduced by adding anti-human IgG (in the example below) specific for HAMA. This type of blocking agent (known as HAMA blockers) is commercially available.
False positive results caused by Fc receptors are also reduced by adding IgG from the animal species in which the secondary antibody is raised.
As illustrated above, more accurate results are obtained by reducing non-specific reactions.