| Code No. |
D263-3 |
|
Anti-CD63 (LAMP-3) (Mouse) mAb
|
| Price |
¥52,800
|
| Size |
100 µL (1 mg/mL) |
| Availability (in Japan) |
10 or more
(In Japan at 17:00, Apr 19, 2024 in JST)
|
| Clonality |
Monoclonal |
| Clone |
R5G2 |
Isotype
(Immunized Animal) |
Rat IgG2b |
| Applications |
WB 2-10 µg/mL
FCM 10 µg/mL (final concentration)
IC* reported. (PMID: 20236428)
Other(Immuno-EM) reported. (PMID: 28956068 / 29669945)
|
Immunogen
(Antigen) |
Mouse bone marrow stroma cell line ST2 |
| Reactivity [Gene ID] |
Mouse[12512] |
| Storage buffer |
1 mg/mL in PBS/50% glycerol, pH 7.2 |
| Storage temp. |
-20°C |
| Conjugate |
Unlabeled |
| Manufacturer |
MBL |
| Alternative names |
ME491, C75951, Tspan30 |
| Background |
CD63 is not only expressed on activated platelets, but also activated monocytes and macrophages, and is weakly expressed on granulocytes, T cell and B cells. It is located on the basophilic granule membranes and translocated to cell surface upon various stimuli. The membrane of lytic granules in CTLs contains CD63/LAMP-3 and other lysosomal-associated glycoproteins (LAMPs) such as CD107a/LAMP-1 and CD107b/LAMP-2. LAMPs have been observed on the cell surface as a result of degranulation. CD63 belongs to a member of the tetraspanin transmembrane-protein (TM4) superfamily, which includes CD9, CD37, CD53, CD81, CD82, CD151 and CD231. Several members of this family form noncovalent associations with integrins, particularly b1 integrins (CD29), and modulate cellular adhesion properties. CD63 has a tyrosine-based internalization motif in the cytoplasmic C-terminal tail and interacts with adaptor protein complexes such as AP-2 and AP-3. Because AP-2 and AP-3 are involved in facilitating the clathrin-mediated endocytosis, CD63 could be directly involved in the internalization of its membrane protein partners. |
| Related products |
MEX002-3 Anti-CD63 (LAMP-3) mAb
MEX001-6 Anti-CD9 mAb-Biotin
MEX002-6 Anti-CD63 (LAMP-3) mAb-Biotin
MEX003-6 Anti-CD81 (TAPA1) mAb-Biotin
MEX001-3 Anti-CD9 mAb
MEX003-3 Anti-CD81 (TAPA1) mAb
MEX002-4 Anti-CD63 (LAMP-3) mAb-FITC
MEX002-12 Anti-CD63 (LAMP-3) mAb-ALP
|
| Product category |
Research area:Immunology Cancer Cell surface antigens Exosomes |
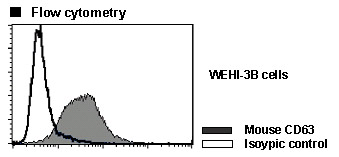
| Data |
|
| Citations |
Western Blotting - Seto S et al. Differential recruitment of CD63 and Rab7-interacting-lysosomal-protein to phagosomes containing Mycobacterium tuberculosis in macrophages. Microbiol Immunol. 54,170-4 (2010)(PMID:20236428)
- Ushio H et al. Crucial role for autophagy in degranulation of mast cells. J Allergy Clin Immunol. 127, 1267-76 (2011)(PMID:21333342)
- Bobrie A et al. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 1, 18397 (2012)(PMID:24009879)
- Padro CJ et al. Adrenergic regulation of IgE involves modulation of CD23 and ADAM10 expression on exosomes. J Immunol. 191, 5383-97 (2013)(PMID:24140643)
- Zonneveld MI et al. Recovery of extracellular vesicles from human breast milk is influenced by sample collection and vesicle isolation procedures. J Extracell Vesicles. 3, 24215 (2014)(PMID:25206958)
- Groot Kormelink T et al. Prerequisites for the analysis and sorting of extracellular vesicle subpopulations by high-resolution flow cytometry. Cytometry A. 89, 135-47 (2016)(PMID:25688721)
- Kowal J et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. PNAS. 113, E968-77 (2016)(PMID:26858453)
- Groot Kormelink T et al. Mast Cell Degranulation Is Accompanied by the Release of a Selective Subset of Extracellular Vesicles That Contain Mast Cell-Specific Proteases. J Immunol. 197, 3382-3392 (2016)(PMID:27619994)
- Nager AR et al. An Actin Network Dispatches Ciliary GPCRs into Extracellular Vesicles to Modulate Signaling. Cell 168, 252-263.e14 (2017)(PMID:28017328)
- Nishida-Aoki N et al. Disruption of Circulating Extracellular Vesicles as a Novel Therapeutic Strategy against Cancer Metastasis. Mol Ther. 25, 181-191 (2017)(PMID:28129113)
- Durcin M et al. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J Extracell Vesicles. 6,1305677 (2017)(PMID:28473884)
- Iraci N et al. Extracellular vesicles are independent metabolic units with asparaginase activity. Nat Chem Biol. 13, 951-955 (2017)(PMID:28671681)
- Laulagnier K et al. Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons. Cell Mol Life Sci. 75, 757-773 (2018)(PMID:28956068)
- Krause M et al. Exosomes as secondary inductive signals involved in kidney organogenesis. J Extracell Vesicles. 7, 1422675 (2018)(PMID:29410779)
- Obata Y et al. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight 3, e99680 (2018)(PMID:29669945)
- Men Y et al. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat Commun. 10, 1038 (2019)(PMID:31515491)
Flow Cytometry - Kunert S et al. The microtubule modulator RanBP10 plays a critical role in regulation of platelet discoid shape and degranulation. Blood 114, 5532-40 (2009)(PMID:19801445)
- Ushio H et al. Crucial role for autophagy in degranulation of mast cells. J Allergy Clin Immunol. 127, 1267-76 (2011)(PMID:21333342)
- Verjan Garcia N et al. SIRPα/CD172a regulates eosinophil homeostasis. J Immunol. 187, 2268-77 (2011)(PMID:21775684)
- Brochetta C et al. Munc18-2 and syntaxin 3 control distinct essential steps in mast cell degranulation. J Immunol. 192, 41-51 (2014)(PMID:24323579)
Immunocytochemistry - Seto S et al. Differential recruitment of CD63 and Rab7-interacting-lysosomal-protein to phagosomes containing Mycobacterium tuberculosis in macrophages. Microbiol Immunol. 54,170-4 (2010)(PMID:20236428)
- Ushio H et al. Crucial role for autophagy in degranulation of mast cells. J Allergy Clin Immunol. 127, 1267-76 (2011)(PMID:21333342)
- Päll T et al. Soluble CD44 interacts with intermediate filament protein vimentin on endothelial cell surface. PLoS One 6, e29305 (2011)(PMID:22216242)
- Lopes da Silva M et al. The host endocytic pathway is essential for Plasmodium berghei late liver stage development. Traffic. 13, 1351-63 (2012)(PMID:22780869)
- Bobrie A et al. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 1, 18397 (2012)(PMID:24009879)
- Krause M et al. Exosomes as secondary inductive signals involved in kidney organogenesis. J Extracell Vesicles. 7, 1422675 (2018)(PMID:29410779)
- Obata Y et al. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight 3, e99680 (2018)(PMID:29669945)
- Tanaka Y et al. Adiponectin promotes muscle regeneration through binding to T-cadherin. Sci Rep. 9, 16 (2019)(PMID:30626897)
Immunohistochemistry - Tanaka Y et al. Adiponectin promotes muscle regeneration through binding to T-cadherin. Sci Rep. 9, 16 (2019)(PMID:30626897)
Other(Immuno-EM) - Laulagnier K et al. Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons. Cell Mol Life Sci. 75, 757-773 (2018)(PMID:28956068)
- Obata Y et al. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight 3, e99680 (2018)(PMID:29669945)
- Nishida K et al. Mast cells play role in wound healing through the ZnT2/GPR39/IL-6 axis. Sci Rep. 9, 10842 (2019)(PMID:31346193)
- Men Y et al. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat Commun. 10, 1038 (2019)(PMID:31515491)
|
 Copyright (C)2015 Medical & Biological Laboratories Co., Ltd. All Rights Reserve
Copyright (C)2015 Medical & Biological Laboratories Co., Ltd. All Rights Reserve